Genomic overlaps
Learning outcomes
To be able to manipulate genomic ranges using the
GRangespackage.To compute statistical significance of overlaps between two sets of genomic ranges using randomization tests, and to understand some of the pitfalls when interpreting such tests.
Table of contents
Setting up
Option A
The easiest way to run this exercise, which we recommend, is on Uppmax using the module system. To do this, start an interactive session like you have done for the previous days, and then do
module load bioinfo-tools
module load R/4.0.0
module load R_packages/4.0.0
module load RStudio
module load samtools
Then set up a working directory, in which you create links to all data files needed for the exercise, e.g. with
cd <some directory where you work with this course>
mkdir -p genomic_overlaps
cd genomic_overlaps
ln -s /sw/courses/epigenomics/genomic_overlaps/* .
Now start R, or rstudio.
Option B
An alternative option, is to run the exercise on Uppmax but in a container instead of using the module system. This is a bit more tricky, but there are several advantages:
You can also use packages that have not previously been installed in Uppmax modules.
You can use the same containers on other computers, and if you use the same container several years from now you are still sure that you are using exactly the same versions of all tools as you did before. This is great for reproducibility. (Please see the course Tools for reproducible research is you are interested in containers, workflows etc.)
To set up this exercise using this option, you have to go through the following steps:
Log in to uppmax, and start an interactive session.
Create a new dirctory for the exercise, and go there
mkdir -p genome_overlaps
cd genome_overlaps
Run the following script on uppmax, to start Rstudio
/sw/courses/epigenomics/genomic_overlaps/startup_script.sh
This script will print some instructions, e.g.
SSH tunnel from your workstation using the following command:
ssh -N -L 8787:r142.uppmax.uu.se:36611 jacke@rackham.uppmax.uu.se
and point your web browser to http://localhost:8787
On your local computer, run the ssh command printed above
Finally, start a web browser on your local computer, and go to http://localhost:8787.
You should now see Rstudio in you web browser, and can start the exercise.
Option C
If this doesn’t work for some reason (e.g. if you don’t have an account on uppmax), you can run the exercise on your laptop. This has been tested on Mac, but chances are that is also works on Linux and Windows. If you haven’t done so for the single cell exercise, first install mamba, and the environment for the labs, following the instructions here.
Then copy the data files for the exercise to your laptop (these files are quite small, altogether 5Mb)
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/beaf32.gff3
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/cp190.gff3
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/ctbp.bed
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/ctcf.gff3
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/dm3_chrom_sizes.txt
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/genes1000bpupstream.bed
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/insv_2_6h.bed
curl -OJ https://export.uppmax.uu.se/naiss2023-23-349/genomic_overlaps/insv_6_12h.bed
Finally, activate the environment and start R or Rstudio, and you are ready to go.
mamba activate environment_epigenomics2023
open -na Rstudio
Introduction to genomic ranges
Genomic regions can be used to represent the locations of many features on the genome, e.g. genes, enchancers, open chromatin, transcripsion factor binding sites, CpG islands, SNPs and more. Handling genomic regions is useful for a wide variety of bioinformatic applications. Some examples are:
Find all promoter regions of a group of genes
What are the closest genes to our transcription factor binding sites?
Which SNPs overlap the binding sites of a transcription factor we are looking at?
How much do our transcription factor binding sites overlap heterochromatin or insulator elements? Do they overlap more than expected by chance?
In R, the GRanges package provides an easy way to work with genomic regions. More information on the GRanges package can be found here.
We will start by looking at some methods to manipulate genomic ranges, using ChIP-seq data from Dai et al. 2013. This study, in Drosophila melanogaster embryos, examined the protein Insensitive (Insv). Two ChIP-seq experiments were made, on 2-6 hour embryos and 6-12 hour embryos. (The peaks for these data sets were called using the Quest peak caller, which reports peaks in a slightly odd format: Here peaks are represented by the 2bp sequences corresponding to the peak summits.)
We start by loading the libraries needed for this exercise.
library("rtracklayer")
library("regioneR")
library("BSgenome.Dmelanogaster.UCSC.dm3")
library("pheatmap")
Next, we load the first Insv data set. We use the function import.bed, which reads a file and returns a GRanges object. This holds information on one or more regions across a genome. Have a look at it. There are corresponding functions to load gff, bw and bedGraph files too. We also use the function filterChromosomes to remove special chromosomes present in the Drosophila genome, e.g. heterochromatin and a special “chromosome” made up of unassembled contigs.
Can you understand the output? How many peaks does this data set have?
set.seed(1234)
insv.2.6 <- import.bed("insv_2_6h.bed")
insv.2.6 <- filterChromosomes(insv.2.6, organism = "dm3")
insv.2.6
head(start(insv.2.6))
head(end(insv.2.6))
head(width(insv.2.6))
sum(width(insv.2.6))
length(insv.2.6)
Operations on a single GRanges object
There are several operations you can perform on a Granges object: shift all regions to the left or right, change their size, get flanking regions, merge overlapping regions, find everything not covered by the regions etc. Try out a few of these. Do you understand what these functions do? Otherwise try e.g. ?flank.
# Get flanking regions
flank(insv.2.6, 10, start=TRUE)
flank(insv.2.6, 10, start=FALSE)
# Shift regions
shift(insv.2.6, 500)
shift(insv.2.6, -500)
# Change size of regions
insv.2.6 + 10
insv.2.6 - 10
# Merge overlapping regions
reduce(insv.2.6 + 249)
# Get everything not covered by the regions
gaps(insv.2.6)
Comparing GRanges
When integrating different data sets, it is often useful to compare several GRanges objects, to see how much they overlap, or where they differ. Here, we load the other Insv data set, from 6-12 hour embryos, and compare it to the data from 2-6 hours. Since each peak is only represented by the 2 bp peak summit, we start by extending the peaks so they are 500bp, and merging overlapping peaks.
Common operations for comparing two GRanges are: union (all sequence covered by any of the GRanges objects), intersect (all sequence covered by both of the GRanges objects), setdiff (all sequence covered by one GRanges object but not the other).
insv.6.12 <- import.bed("insv_6_12h.bed")
insv.6.12 <- filterChromosomes(insv.6.12, organism = "dm3")
insv.2.6 <- reduce(insv.2.6 + 249)
insv.6.12 <- reduce(insv.6.12 + 249)
insv.2.6
insv.6.12
# Union
union(insv.2.6, insv.6.12)
union(insv.2.6, gaps(insv.2.6))
# How many bp are present in each peaks set? In any of the peak sets?
sum(width(insv.2.6))
sum(width(insv.6.12))
sum(width(union(insv.2.6, insv.6.12)))
# Intersection
intersect(insv.2.6, insv.6.12)
# How many bp are present in both peak sets?
sum(width(intersect(insv.2.6, insv.6.12)))
# Setdiff
setdiff(insv.2.6, insv.6.12)
# How many bp are prsent in the 2-6 hour peak set, but not in the 6-12 hour peak set?
sum(width(setdiff(insv.2.6, insv.6.12)))
# Can you figure out what this means?
sum(width(setdiff(insv.2.6, insv.6.12))) + sum(width(intersect(insv.2.6, insv.6.12)))
If we have two data sets, the function findOverlaps calculates which of the regions in the two data sets overlap.
# Which regions overlap?
ol <- findOverlaps(insv.2.6, insv.6.12)
ol
# One range in object A may overlap several ranges in object B, and the other way around
which(duplicated(queryHits(ol)))
Statistical significance of overlaps
Determining if two sets of genomic regions overlap more than expected by chance is a common problem in genomics. Here we will try the regioneR package to do this, but there are many other methods available. As you will sees this is a quite difficult problem, and the results should be interpreted with caution.
The regioneR package uses randomization tests to asses how significant the overlap between two data sets is. The basic idea is to:
Calulate the overlap between the two given data sets A and B
Create a randomized data set B’, with the same number of regions, and the same sizes as B, distributed randomly across the genome.
Calculate the overlap between A and B’.
Repeat steps 2 and 3 many times (e.g. 1000) to get a distribution of how many overlaps we would expect by chance.
Compare the results from step 1 (A vs B) to the results for all the randomized data sets. This makes it possible to calculate a p-value and a z-score.
To illustrate this, we create a data set of 100 regions, randomly ditributed across the genome. Using the test with 100 randomizations, we check if there is a significant overlap between the Insv peaks and our random data set. How do you interpret the results?
# Read sizes of chromosomes
dm3 <- read.table("dm3_chrom_sizes.txt")
dm3 <- filterChromosomes(getGenome(dm3), organism="dm3")
rr <- createRandomRegions(nregions=100, length.mean=500, length.sd=0, genome=dm3)
permRes <- overlapPermTest(A=rr, B=insv.2.6, ntimes=100, alternative="greater", genome=getGenome(dm3), mc.set.seed=FALSE, verbose=TRUE)
summary(permRes)
plot(permRes)
Using the same strategy, we can check if there is a significant overlap between the two Insv data sets, from different time points. How do you interpret the results?
permRes <- overlapPermTest(A=insv.2.6, B=insv.6.12, ntimes=100, alternative="greater", genome=getGenome(dm3), mc.set.seed=FALSE, verbose=TRUE)
summary(permRes)
plot(permRes)
Example with insulator elements
In Dai et al. 2015 it was shown that Insv often binds together with insulator proteins (BEAF-32, CP190, CTCF and others) across the Drosophila genome.
Insulator elements were first described as DNA elements that can restrict e.g. interactions between enhancers and target genes or the spread of heterochromatin.
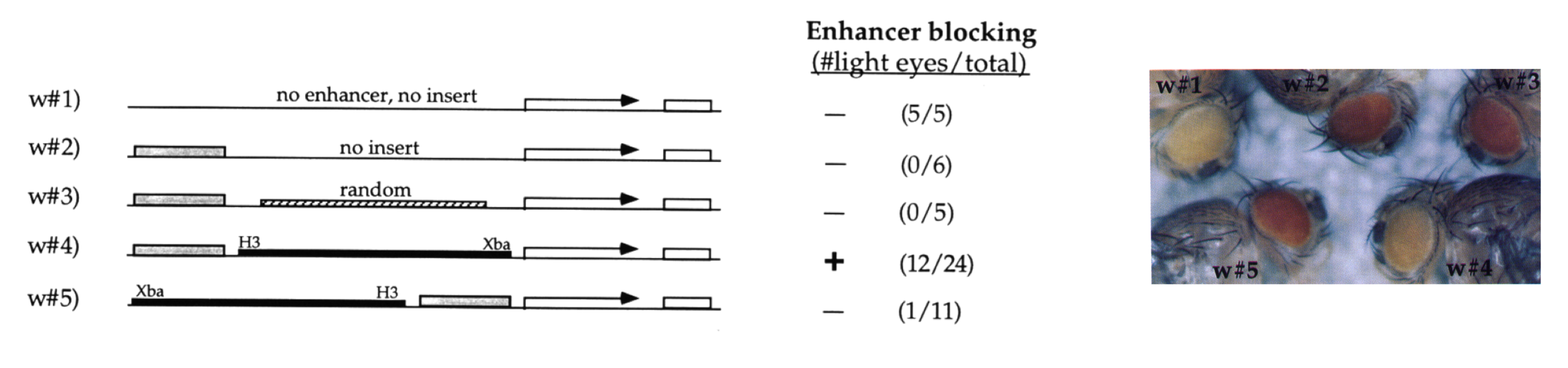
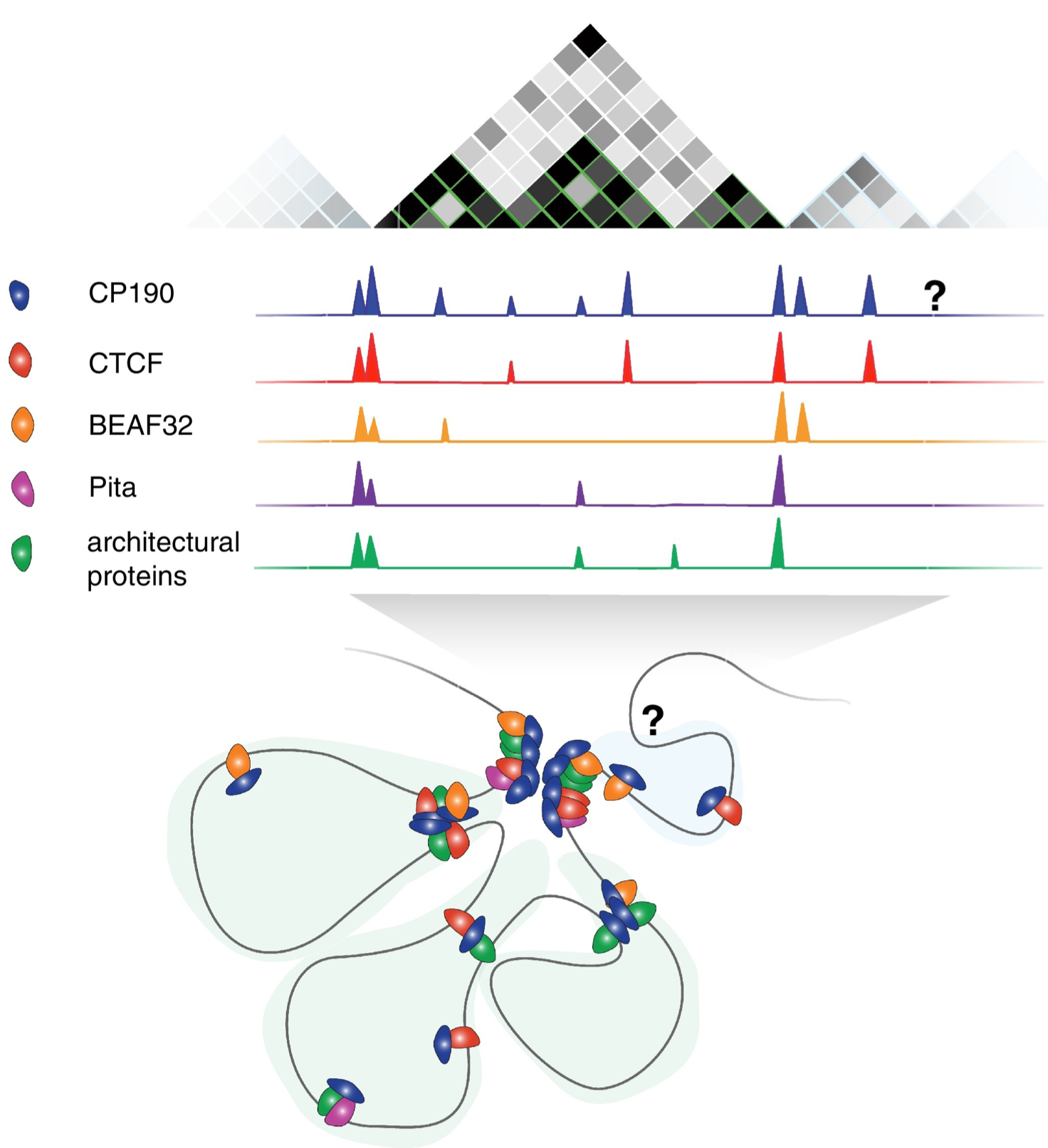
Later it was found that insulator elements control DNA looping, so that enhancers and target genes can end up in different loop domains (≈ topologically associated domains, TADs). Image taken from this review.
To check if Insv overlaps insulator elements, we use some public data on insulator proteins, and compare these to the Insv regions. We start by loading and formatting the data.
beaf32 <- import.gff3("beaf32.gff3")
cp190 <- import.gff3("cp190.gff3")
ctcf.1 <- import.gff3("ctcf.gff3")
allDataSets <- list(insv.2.6=insv.2.6,
insv.6.12=insv.6.12,
beaf32=beaf32,
cp190=cp190,
ctcf.1=ctcf.1)
# These data sets are from different studies, and on different formats.
# We have to make sure that they are all on the same format, so that all chromosome names match.
lapply(allDataSets, seqlevelsStyle)
for(i in 1:length(allDataSets)){
seqlevelsStyle(allDataSets[[i]])<-"UCSC"
allDataSets[[i]] <- filterChromosomes(allDataSets[[i]], organism = "dm3")
}
lapply(allDataSets, seqlevelsStyle)
Then, we simply count the overlaps between the various data sets. We loop through all pairs of data sets i,j, and count the overlaps. In the matrix below each, entry i,j, represents the fraction of regions in data set i that overlap with data set j. We also plot the results as a heat map, where different levels of overlap are represented by different colors. Does Insv overlap a lot with the insulator proteins?
overlapFraction <- matrix(NA, nrow=length(allDataSets), ncol=length(allDataSets))
rownames(overlapFraction) <- names(allDataSets)
colnames(overlapFraction) <- names(allDataSets)
for(i in 1:length(allDataSets)){
for(j in 1:length(allDataSets)){
overlapFraction[i,j] <- numOverlaps(A=allDataSets[[i]], B=allDataSets[[j]])/length(allDataSets[[i]])
}
}
round(overlapFraction,2)
useCols <- colorRampPalette(c("white", "red"))(100)
pheatmap(overlapFraction, cluster_rows=FALSE, cluster_cols=FALSE, color = useCols, main="% overlap")
For comparison, we add data on an unrelated transcription factor, CtBP, and re-run the analysis. How much does Insv overlap the insulator proteins BEAF-32, CP190 and CTCT, compared to CtBP?
# Load and format CtBp data
ctbp <- import.bed("ctbp.bed")
seqlevelsStyle(ctbp)<-"UCSC"
ctbp <- filterChromosomes(ctbp, organism = "dm3")
allDataSets <- c(allDataSets, ctbp=ctbp)
# Re-run overlap analysis
overlapFraction <- matrix(NA, nrow=length(allDataSets), ncol=length(allDataSets))
rownames(overlapFraction) <- names(allDataSets)
colnames(overlapFraction) <- names(allDataSets)
for(i in 1:length(allDataSets)){
for(j in 1:length(allDataSets)){
overlapFraction[i,j] <- numOverlaps(A=allDataSets[[i]], B=allDataSets[[j]])/length(allDataSets[[i]])
}
}
round(overlapFraction,2)
useCols <- colorRampPalette(c("white", "red"))(100)
pheatmap(overlapFraction, cluster_rows=FALSE, cluster_cols=FALSE, color = useCols, main="% overlap")
We can now check if the overlaps between the different proteins are more common than would be expected by chance. Again, we loop through all pairs of data sets i,j, but this time we do a randomization test for each pair i,j, and save the p-values and z-scores. Because this takes a while to run, we only use 50 randomization rounds. (To reduce runtime even more, we only compare each pair of data sets once and don’t compare data sets to themselves.)
Looking at the p-values is not very informative, since they are all quite low. To get even lower p-values, we would have to run a lot more randomization rounds, which takes time. (P-values of 1e5 would require 100000 randomizations.) In this case, Z-scores are more informative, since they can provide a measure of how much bigger the overlap between data sets is, compared to randomized data.
overlapP <- matrix(NA, nrow=length(allDataSets), ncol=length(allDataSets))
rownames(overlapP) <- names(allDataSets)
colnames(overlapP) <- names(allDataSets)
overlapZ <- matrix(NA, nrow=length(allDataSets), ncol=length(allDataSets))
rownames(overlapZ) <- names(allDataSets)
colnames(overlapZ) <- names(allDataSets)
nTimes <- 50
for(i in 1:length(allDataSets)){
for(j in 1:length(allDataSets)){
if(i<j){
permRes <- overlapPermTest(A=allDataSets[[i]],
B=allDataSets[[j]],
ntimes=nTimes,
alternative="greater",
genome=getGenome(dm3),
mc.set.seed=FALSE,
verbose=FALSE)
overlapP[i,j] <- permRes$numOverlaps$pval
overlapZ[i,j] <- permRes$numOverlaps$zscore
}
}
}
# Fill in the missing values.
for(i in 1:length(allDataSets)){
for(j in 1:length(allDataSets)){
if(i>j){
overlapP[i,j] <- overlapP[j,i]
overlapZ[i,j] <- overlapZ[j,i]
}
}
}
diag(overlapP) <- 0
diag(overlapZ) <- NA
round(overlapP,2)
round(overlapZ,2)
useCols <- colorRampPalette(c("white", "red"))(100)
pheatmap(overlapP, cluster_rows=FALSE, cluster_cols=FALSE, color = useCols, main="P-values")
pheatmap(overlapZ, cluster_rows=FALSE, cluster_cols=FALSE, color = useCols, main="z-scores")
Different sampling methods
When testing if the overlap between two data sets is significant, there are many choices that could have a big impact on the results. For example, most transcription factor binding sites are enriched around transcription start sites (TSS). This means that if we use a randomization strategy where we can place regions over the entire genome, we might overestimate the significance of the overlaps.
Below, we check whether the results change if we instead focus on promoter regions only. For this, we load coordinates of promoter regions, here defined as [TSS-1500, TSS+500]. We then only consider binding sites that fall within the promoter regions. As you can see, we still retain most binding sites. In this re-sampling strategy, we now only sample promoter regions instead of sampling random regions across the genome. How does this affect the results?
(Since this randomization strategy is faster than sampling random regions, we now use 500 randomization rounds.)
# Load promoter regions
us <- import.bed("genes1000bpupstream.bed") + 500
seqlevelsStyle(us)<-"UCSC"
us <- filterChromosomes(us, organism = "dm3")
# Only use peaks within promoter regions
allDataSetsUs <- lapply(allDataSets, function(x){GenomicRanges::intersect(x,us,ignore.strand=TRUE) })
lapply(allDataSetsUs , length)
# Re-run ovlerap analysis
overlapP <- matrix(NA, nrow=length(allDataSets), ncol=length(allDataSets))
rownames(overlapP) <- names(allDataSets)
colnames(overlapP) <- names(allDataSets)
overlapZ <- matrix(NA, nrow=length(allDataSets), ncol=length(allDataSets))
rownames(overlapZ) <- names(allDataSets)
colnames(overlapZ) <- names(allDataSets)
nTimes <- 500
for(i in 1:length(allDataSets)){
for(j in 1:length(allDataSets)){
if(i<j){
permRes <- permTest(A=allDataSets[[i]],
B=allDataSets[[j]],
ntimes=nTimes,
randomize.function=resampleRegions,
universe=us,
alternative="greater", evaluate.function=numOverlaps,
count.once=TRUE, mc.set.seed=FALSE, mc.cores=4)
overlapP[i,j] <- permRes$numOverlaps$pval
overlapZ[i,j] <- permRes$numOverlaps$zscore
}
}
}
# Fill in the missing values.
for(i in 1:length(allDataSets)){
for(j in 1:length(allDataSets)){
if(i>j){
overlapP[i,j] <- overlapP[j,i]
overlapZ[i,j] <- overlapZ[j,i]
}
}
}
diag(overlapP) <- 0
diag(overlapZ) <- NA
round(overlapP,3)
round(overlapZ,2)
useCols <- colorRampPalette(c("white", "red"))(100)
pheatmap(overlapP, cluster_rows=FALSE, cluster_cols=FALSE, color = useCols, main="P-values")
pheatmap(overlapZ, cluster_rows=FALSE, cluster_cols=FALSE, color = useCols, main="z-scores")